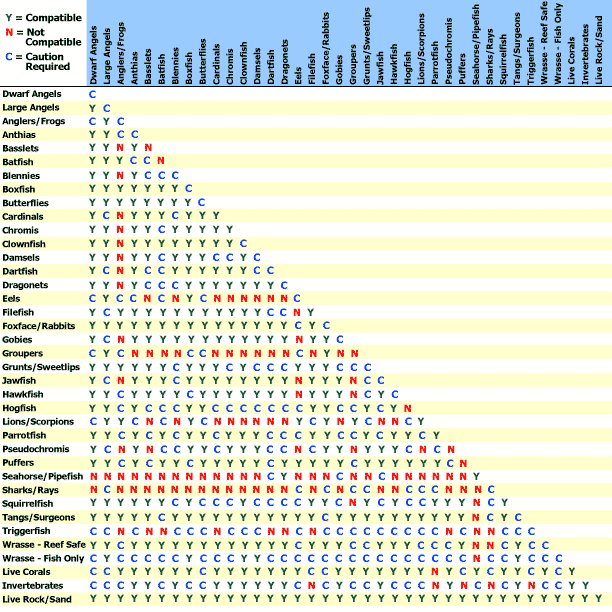
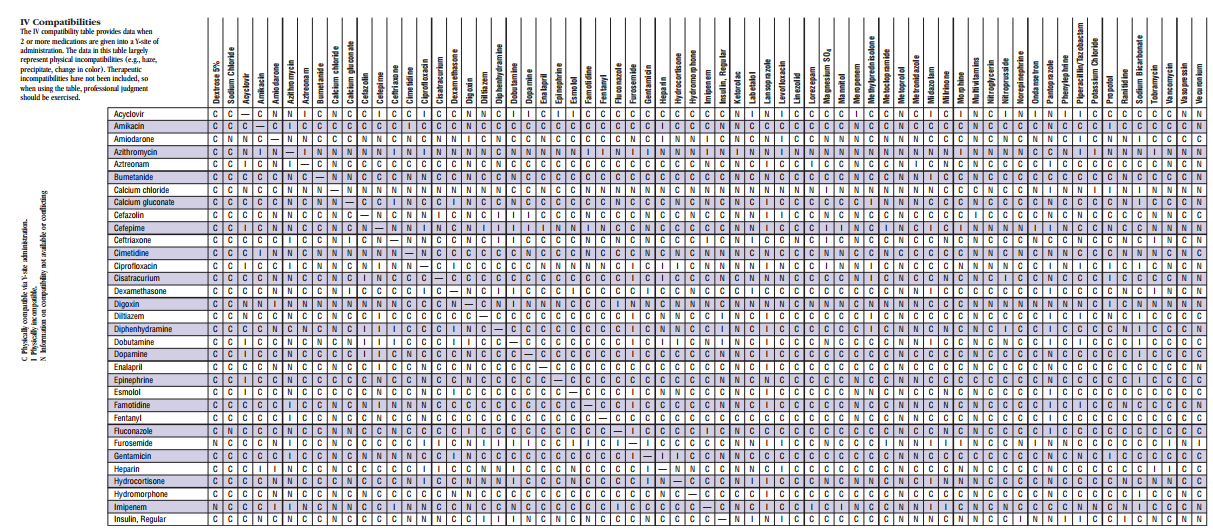
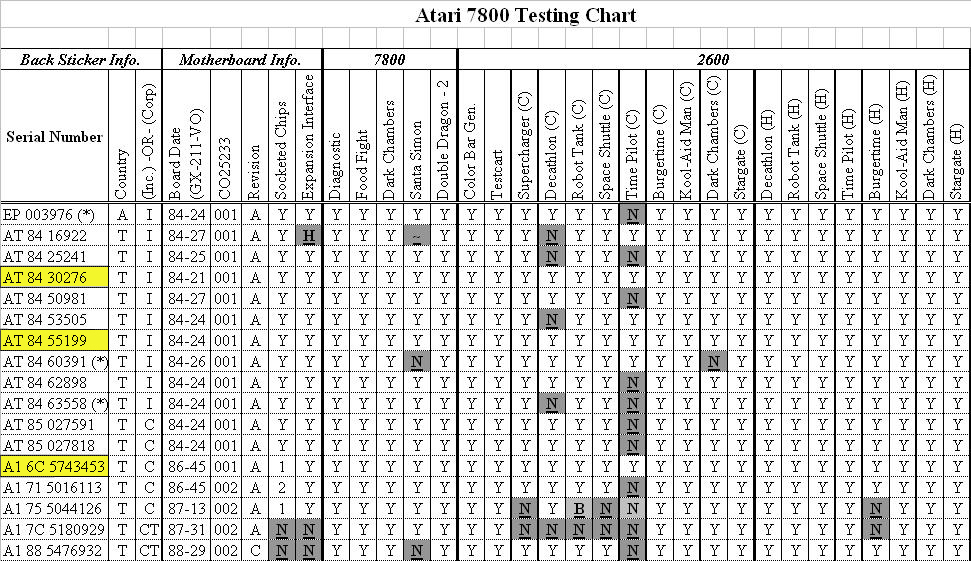
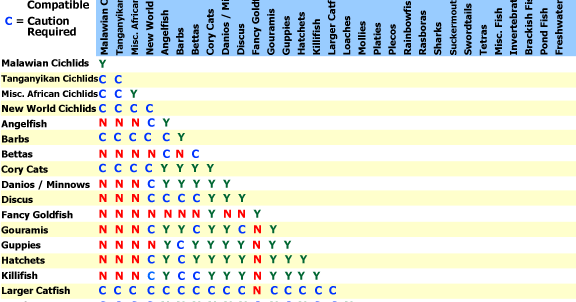
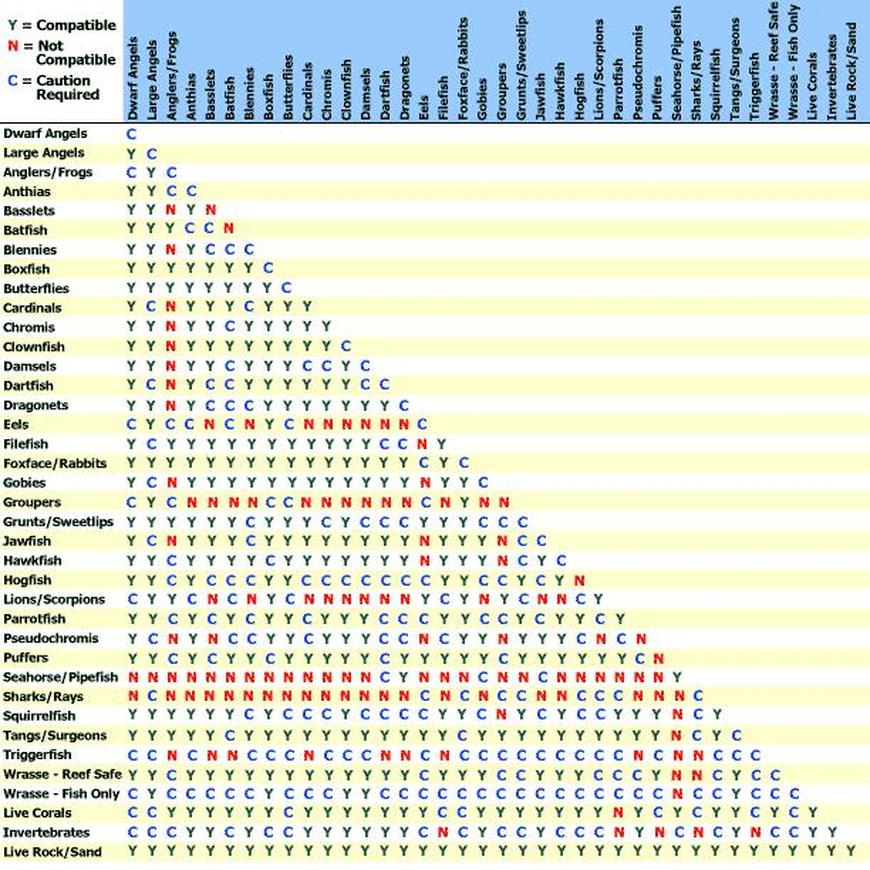
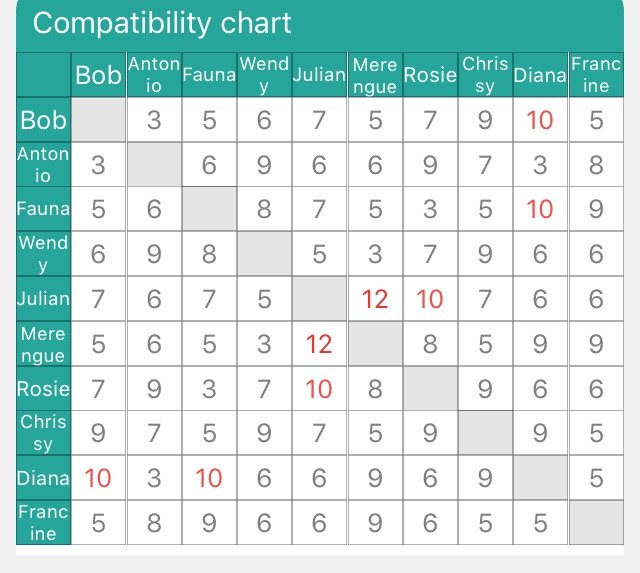
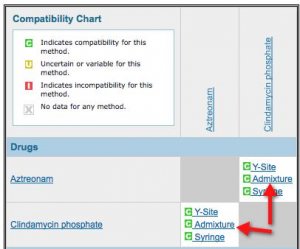
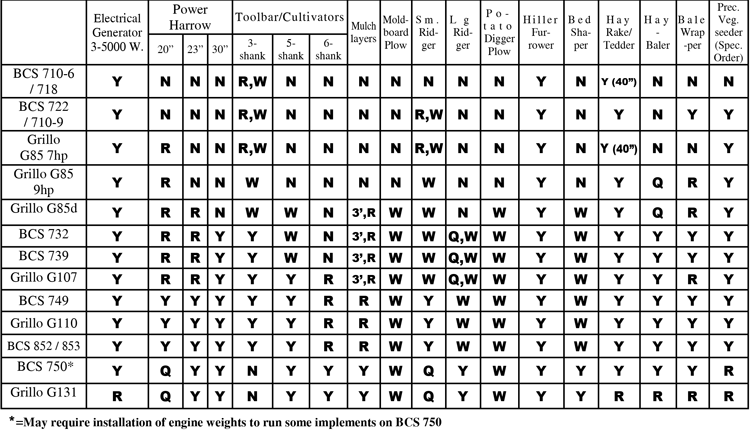
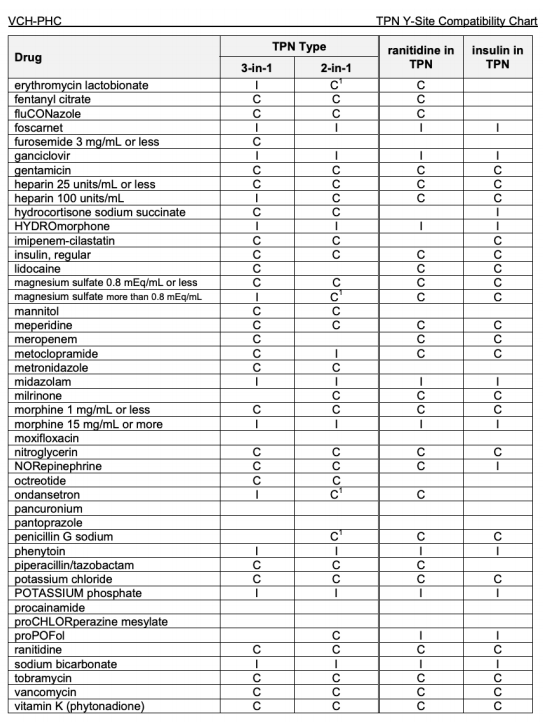
Y Site Compatibility Chart

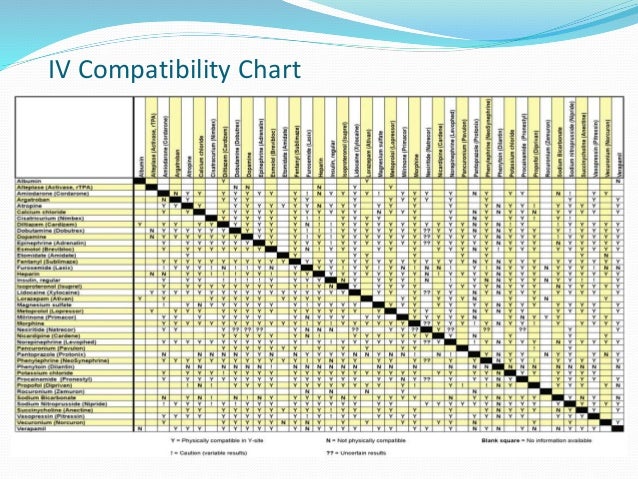
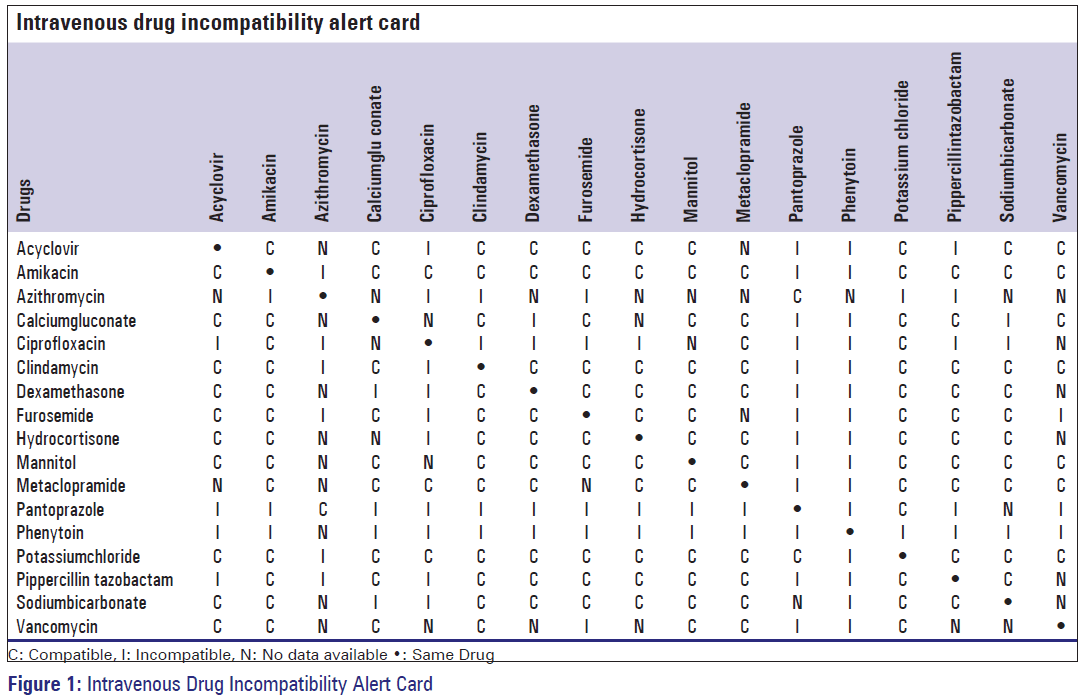
Features a compatibility chart covering over 100 drugs.
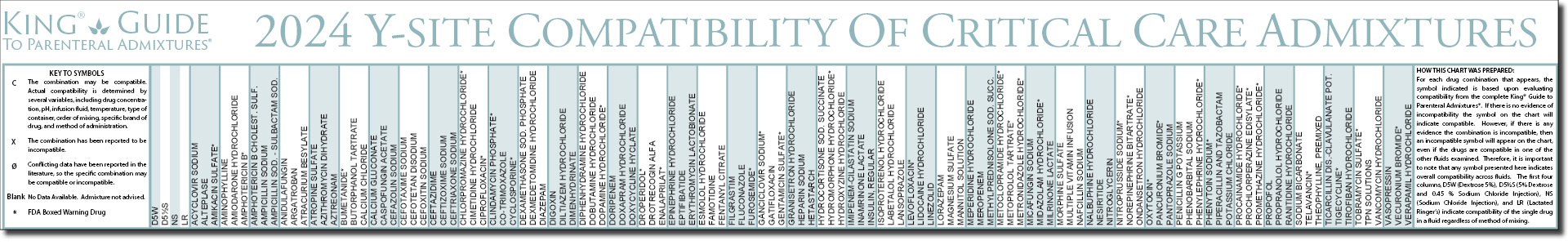
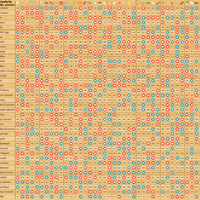
Y site compatibility chart. With 122 iv drugs and 4 fluids the wall charts provide a comprehensive view of y site compatibility for critical care admixtures and chemotherapy admixtures on a 27 x 27 wall chart. Y site compatibility wall charts. The king guide wall charts are a useful reference to have visible in the hospital and pharmacy for situations where a quick yes or no answer is needed.
Y site compatibility wall chartscritical information at the point of care. When this occurred incompatible was used. Fda revokes covid 19 eua for hydroxychloroquine and chloroquine.
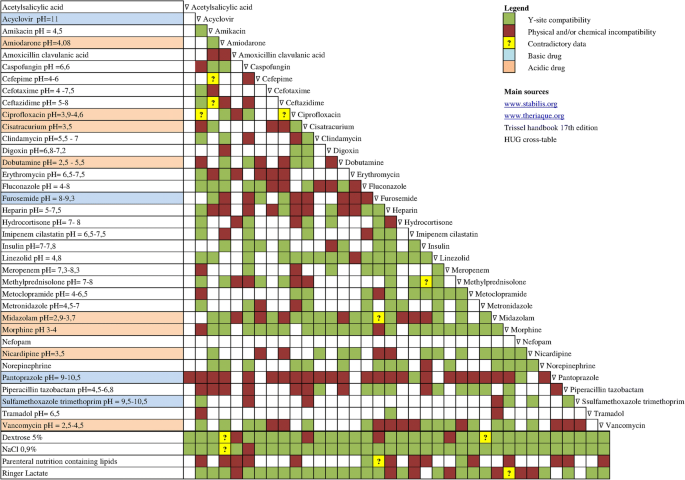
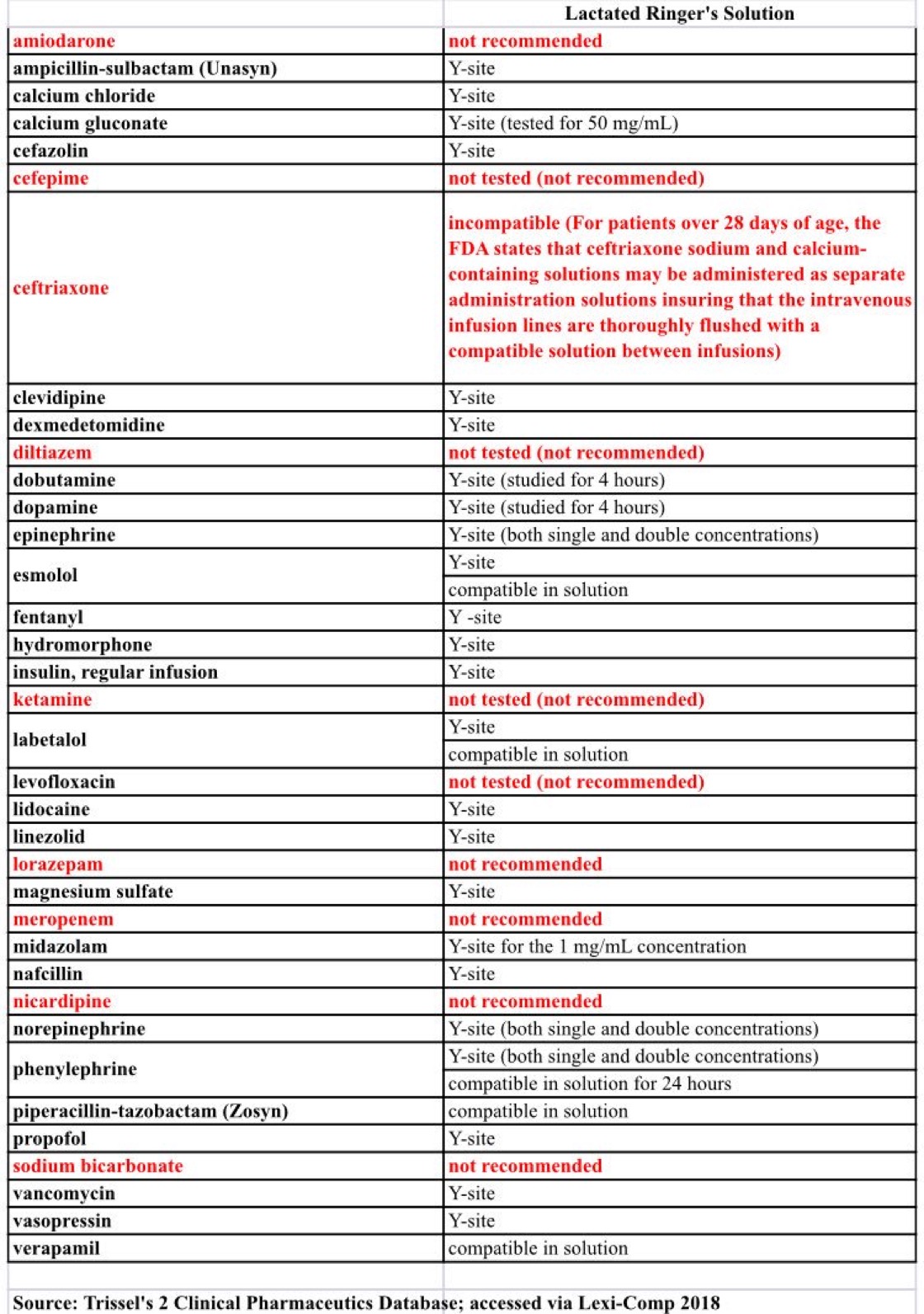
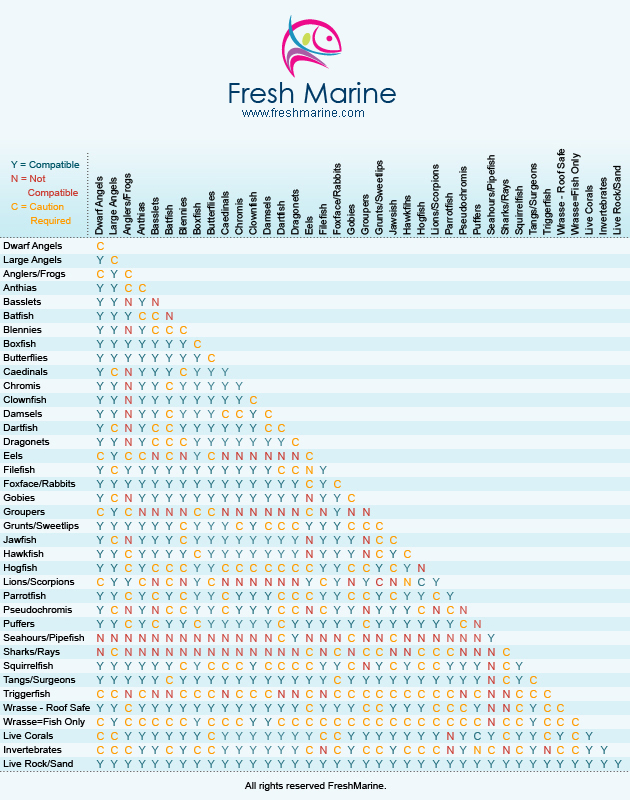
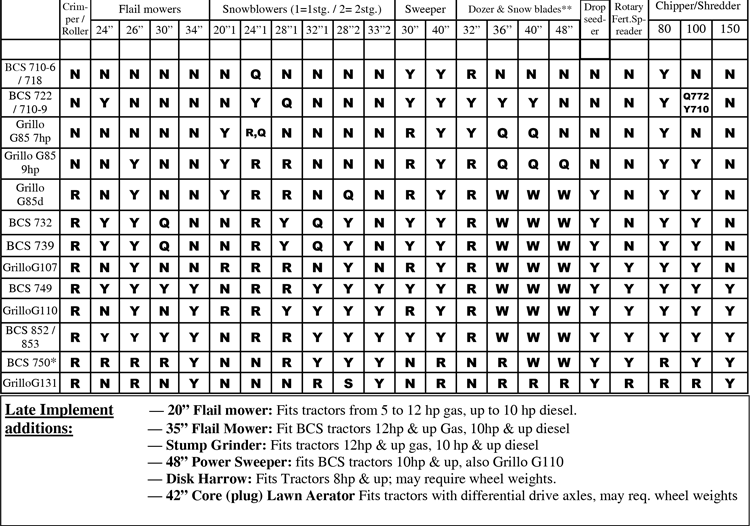
Vch phc tpn y site compatibility chart 1 the piggy back infusion of lipid emulsion if any should be interrupted during co infusion of the 2 in 1 tpn with this medication 3 in1 travasol dextrose lipid emulsion additives all in one bag 2 in 1 travasol dextrose additives in one bag lipid bag administered separately via piggy back. Drugs in syringe compatibility y site injection compatibility 1 1 mixture additionally in some cases one brand of product may be compatible but another brand of drug is not. Click the image above to see a larger image of a portion of this wall chart.
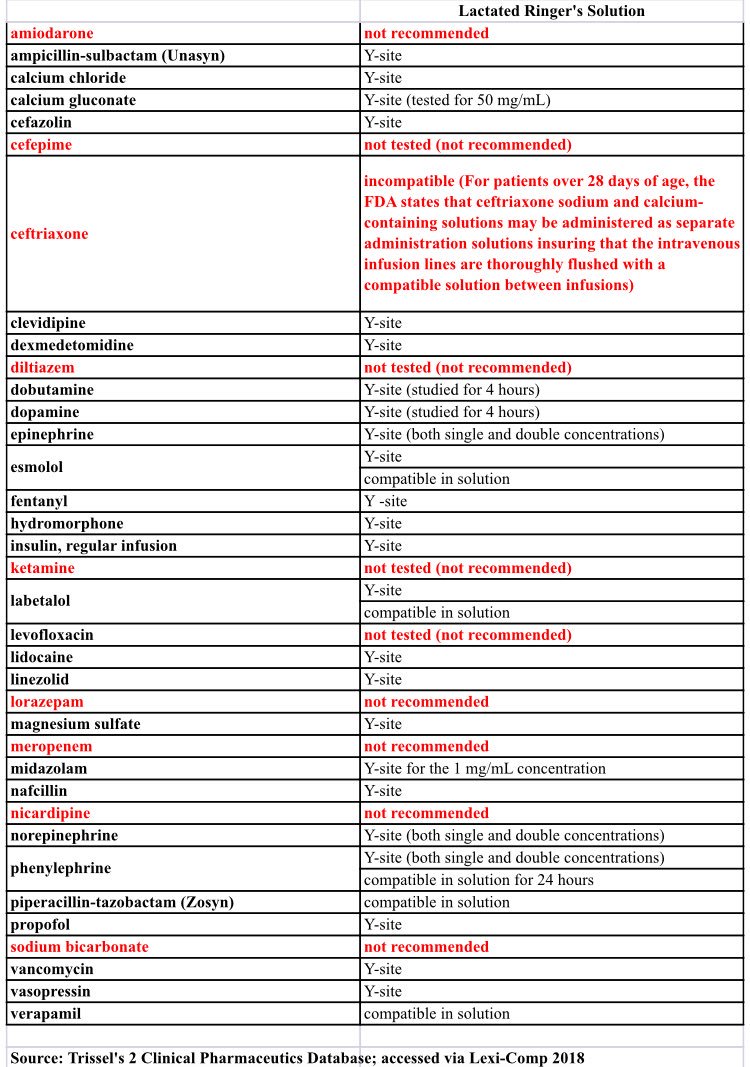
Amiodarone ampicillin calcium chloride calcium gluconate cefazolin ceftazidime ceftriaxone cefuroxime ciprofloxacin clindamycin cyclosporine dobutamine dopamine epinephrine fentanyl fluconazole furosemide gentamicin heparin hydralazine hydrocortisone sodium succinate hydromorphone insulin regular human ketamine labetalol lidocaine magnesium sulfate meropenem metronidazole midazolam milrinone. Y site injection drug compatibility chart for critical care wards. The 2020 y site compatibility of critical care admixtures wall chart reports on the compatibility of iv drugs commonly administered to patients in a critical care setting and includes antibiotics vitamins tpn and other drugs.
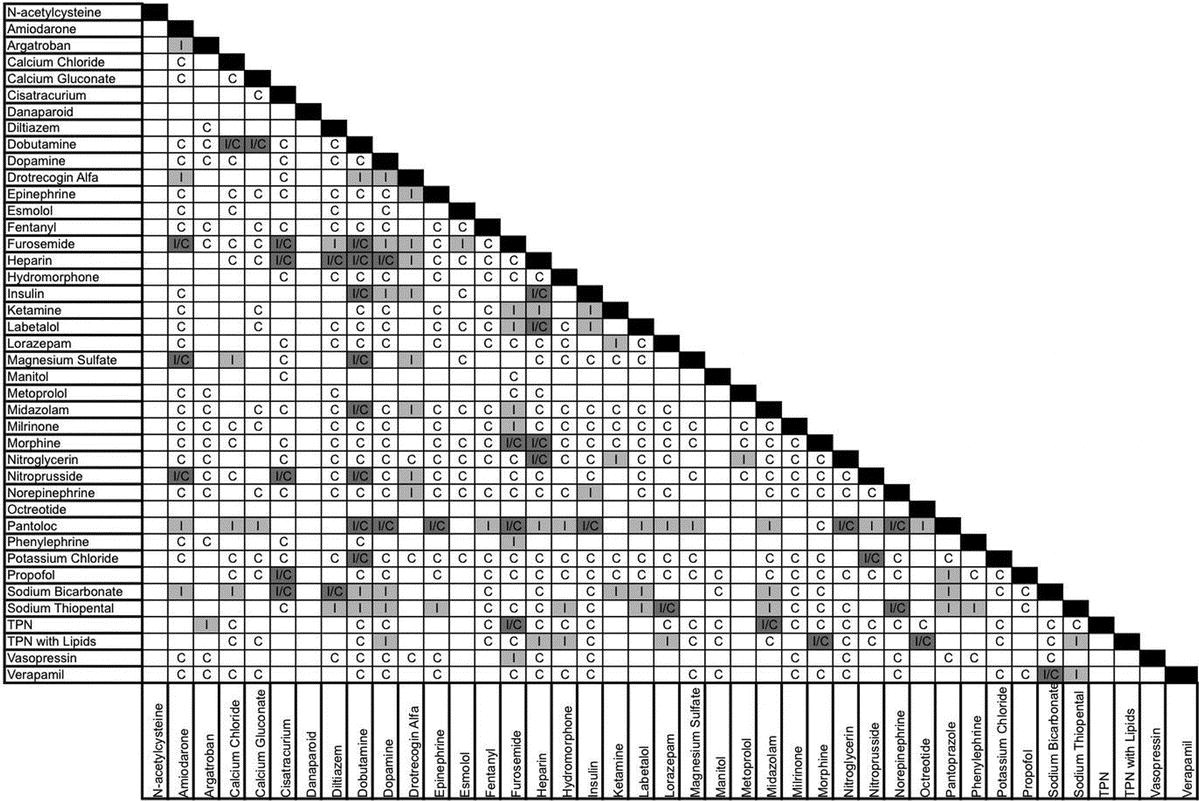
Thames valley critical care network pharmacists group 10 november 2005 milrinone rocuronium salbutamol omeprazole potassium phosphate remifentanil vasopressin arginine vasopressin vecuronium secretarial support was provided by sue gurney thames valley y site intravenous drugs compatibility chart november 2005. On june 15 2020 the us fda revoked the emergency use authorization eua that allowed for chloroquine phosphate and hydroxychloroquine sulfate donated to the strategic national stockpile to be used to treat certain hospitalized patients with covid 19 when a clinical trial was unavailable or participation in a clinical trial.